Genetically Encoded Azulenylalanine Reveals Vibrational Energy Transfer in Proteins
Professor Ned Budisa and Prof. Jens Bredenbeck from Goethe University in Frankfurt, Germany join their efforts to provide experimental evidence for vibrational energy transfer (VET) in proteins between allosterically communicating sites. In the last decade, considerable efforts have been made to understand the biophysical foundations of allostery, a ubiquitous phenomenon in functional proteins. While in computer simulations, mapping energy transfer pathways is straightforward, experimental evidence for these pathways between allosteric sites is still missing due to the lack of suitable spectroscopic tools for their direct observation.
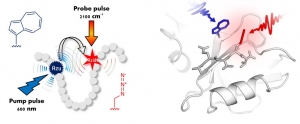
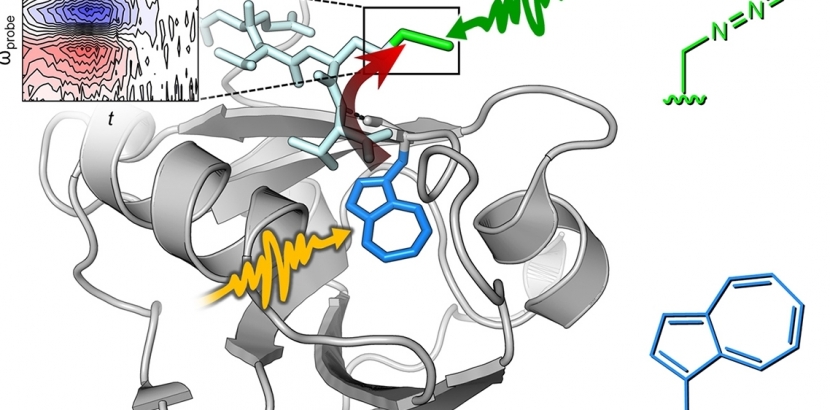
Professors Ned Budisa and Jens Bredenbeck solved this problem by a precise installation of the tryptophan analog β-(1-azulenyl)-L-alanine (AzAla) at defined positions in target proteins via genetic code expansion. In addition, a high-resolution crystal structure of the aminoacyl-tRNA synthetase, the enzyme activating AzAla, determined by Prof. Holger Dobbek and Dr. Berta Martins from the Humboldt University in Berlin, Germany.
The azulene moiety of AzAla carries unique spectroscopic properties. Upon excitation with blue light, it serves as a fluorescence label, whereas upon excitation with orange light, azulene releases the absorbed photon energy as vibrational energy in a split picosecond. In particular, the synthetic amino acid absorbs light at 600 nm and effectively converts that energy to heat, a process known as vibrational energy transfer (VET), which is monitored by ultrafast IR spectroscopy. This makes AzAla the key tool for VET measurements in proteins.
In a recent Angewandte Chemie publication, Bredenbeck and Budisa share their findings which will open new research avenues towards elucidating paths of biological information and energy transfer in protein structures. This will enable a deeper understanding of the fundamental principles behind allosteric regulation of protein activity, active site energy dissipation and enzyme catalysis.
Graphics prepared by Dr.Vladimir Kubyshkin and Dr. Tobias Baumann
For more information please visit: